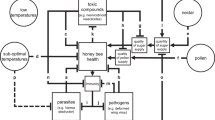
Abstract
Global concern over widely documented declines in pollinators1,2,3 has led to the identification of anthropogenic stressors that, individually, are detrimental to bee populations4,5,6,7. Synergistic interactions between these stressors could substantially amplify the environmental effect of these stressors and could therefore have important implications for policy decisions that aim to improve the health of pollinators3,8,9. Here, to quantitatively assess the scale of this threat, we conducted a meta-analysis of 356 interaction effect sizes from 90 studies in which bees were exposed to combinations of agrochemicals, nutritional stressors and/or parasites. We found an overall synergistic effect between multiple stressors on bee mortality. Subgroup analysis of bee mortality revealed strong evidence for synergy when bees were exposed to multiple agrochemicals at field-realistic levels, but interactions were not greater than additive expectations when bees were exposed to parasites and/or nutritional stressors. All interactive effects on proxies of fitness, behaviour, parasite load and immune responses were either additive or antagonistic; therefore, the potential mechanisms that drive the observed synergistic interactions for bee mortality remain unclear. Environmental risk assessment schemes that assume additive effects of the risk of agrochemical exposure may underestimate the interactive effect of anthropogenic stressors on bee mortality and will fail to protect the pollinators that provide a key ecosystem service that underpins sustainable agriculture.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data used in this analysis are available at OSF (https://osf.io/8xnua/).
Code availability
All code used in this analysis is available at OSF (https://osf.io/8xnua/).
References
Holden, C. Report warns of looming pollination crisis in North America. Science 314, 397 (2006).
Aizen, M. A. & Harder, L. D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 19, 915–918 (2009).
Goulson, D., Nicholls, E., Botías, C. & Rotheray, E. L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 (2015).
Woodcock, B. A. et al. Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat. Commun. 7, 12459 (2016).
Siviter, H., Brown, M. J. F. & Leadbeater, E. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 561, 109–112 (2018).
Cameron, S. A. et al. Patterns of widespread decline in North American bumble bees. Proc. Natl Acad. Sci. USA 108, 662–667 (2011).
Powney, G. D. et al. Widespread losses of pollinating insects in Britain. Nat. Commun. 10, 1018 (2019).
Vanbergen, A. J. & The Insect Pollinators Initiative. Threats to an ecosystem service: pressures on pollinators. Front. Ecol. Environ. 11, 251–259 (2013).
EFSA. Bee health. https://www.efsa.europa.eu/en/topics/topic/bee-health (2019).
Foley, J. A. et al. Global consequences of land use. Science 309, 570–574 (2005).
Tilman, D., Cassman, K. G., Matson, P. A., Naylor, R. & Polasky, S. Agricultural sustainability and intensive production practices. Nature 418, 671–677 (2002).
Potts, S. G. et al. Safeguarding pollinators and their values to human well-being. Nature 540, 220–229 (2016).
Pettis, J. S. et al. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE 8, e70182 (2013).
Siviter, H., Folly, A. J., Brown, M. J. F. & Leadbeater, E. Individual and combined impacts of sulfoxaflor and Nosema bombi on bumblebee (Bombus terrestris) larval growth. Proc. R. Soc. B 287, 20200935 (2020).
Retschnig, G. et al. Effects, but no interactions, of ubiquitous pesticide and parasite stressors on honey bee (Apis mellifera) lifespan and behaviour in a colony environment. Environ. Microbiol. 17, 4322–4331 (2015).
Doublet, V., Labarussias, M., de Miranda, J. R., Moritz, R. F. A. & Paxton, R. J. Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 17, 969–983 (2015).
Folt, C. L., Chen, C. Y., Moore, M. V. & Burnaford, J. Synergism and antagonism among multiple stressors. Limnol. Oceanogr. 44, 864–877 (1999).
Di Prisco, G. et al. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl Acad. Sci. USA 110, 18466–18471 (2013).
Collison, E., Hird, H., Cresswell, J. & Tyler, C. Interactive effects of pesticide exposure and pathogen infection on bee health – a critical analysis. Biol. Rev. Camb. Philos. Soc. 91, 1006–1019 (2016).
Tsvetkov, N. et al. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 356, 1395–1397 (2017).
Carnesecchi, E. et al. Investigating combined toxicity of binary mixtures in bees: meta-analysis of laboratory tests, modelling, mechanistic basis and implications for risk assessment. Environ. Int. 133 (Pt B), 105256 (2019).
Jackson, M. C., Loewen, C. J. G., Vinebrooke, R. D. & Chimimba, C. T. Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob. Change Biol. 22, 180–189 (2016).
Piggott, J. J., Townsend, C. R. & Matthaei, C. D. Reconceptualizing synergism and antagonism among multiple stressors. Ecol. Evol. 5, 1538–1547 (2015).
Ascher, J. S. & Pickering, J. Discover life: bee species guide and world checklist (Hymenoptera: Apoidea: Anthophila). https://www.discoverlife.org/mp/20q?guide=Apoidea_species&flags=HAS (2012).
Gill, R. J., Ramos-Rodriguez, O. & Raine, N. E. Combined pesticide exposure severely affects individual- and colony-level traits in bees. Nature 491, 105–108 (2012).
Schmid-Hempel, P. Evolutionary Parasitology (Oxford Univ. Press, 2011).
Sánchez-Bayo, F. et al. Are bee diseases linked to pesticides? — A brief review. Environ. Int. 89–90, 7–11 (2016).
Brandt, A., Gorenflo, A., Siede, R., Meixner, M. & Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 86, 40–47 (2016).
Vaudo, A. D., Patch, H. M., Mortensen, D. A., Tooker, J. F. & Grozinger, C. M. Macronutrient ratios in pollen shape bumble bee (Bombus impatiens) foraging strategies and floral preferences. Proc. Natl Acad. Sci. USA 113, E4035–E4042 (2016).
Fürst, M. A., McMahon, D. P., Osborne, J. L., Paxton, R. J. & Brown, M. J. F. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 506, 364–366 (2014).
Cedergreen, N. Quantifying synergy: a systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE 9, e96580 (2014).
Carvell, C. et al. Declines in forage availability for bumblebees at a national scale. Biol. Conserv. 132, 481–489 (2006).
Baude, M. et al. Historical nectar assessment reveals the fall and rise of floral resources in Britain. Nature 530, 85–88 (2016).
Ovaskainen, O. et al. Community-level phenological response to climate change. Proc. Natl Acad. Sci. USA 110, 13434–13439 (2013).
Carvell, C. et al. Bumblebee family lineage survival is enhanced in high-quality landscapes. Nature 543, 547–549 (2017).
Siviter, H. & Muth, F. Do novel insecticides pose a threat to beneficial insects? Proc. R. Soc. B 287, 20201265 (2020).
Topping, C. J., Aldrich, A. & Berny, P. Overhaul environmental risk assessment for pesticides. Science 367, 360–363 (2020).
Sgolastra, F. et al. Bees and pesticide regulation: lessons from the neonicotinoid experience. Biol. Conserv. 241, 108356 (2020).
Mullin, C. A. Effects of ‘inactive’ ingredients on bees. Curr. Opin. Insect Sci. 10, 194–200 (2015).
Colin, T., Monchanin, C., Lihoreau, M. & Barron, A. B. Pesticide dosing must be guided by ecological principles. Nat. Ecol. Evol. 4, 1575–1577 (2020).
Milner, A. M. & Boyd, I. L. Toward pesticidovigilance. Science 357, 1232–1234 (2017).
Franklin, E. L. & Raine, N. E. Moving beyond honeybee-centric pesticide risk assessments to protect all pollinators. Nat. Ecol. Evol. 3, 1373–1375 (2019
Brühl, C. A. & Zaller, J. G. Biodiversity decline as a consequence of an inappropriate environmental risk assessment of pesticides. Front. Environ. Sci. 7, 177 (2019).
OECD. Test No. 245: Honey Bee (Apis Mellifera L.), Chronic Oral Toxicity Test (10-Day Feeding) (OECD, 2017).
Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48 (2010).
Duval, S. & Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56, 455–463 (2000).
Woodcock, B. A. et al. Meta-analysis reveals that pollinator functional diversity and abundance enhance crop pollination and yield. Nat. Commun. 10, 1481 (2019).
Siviter, H., Koricheva, J., Brown, M. J. F. & Leadbeater, E. Quantifying the impact of pesticides on learning and memory in bees. J. Appl. Ecol. 55, 2812–2821 (2018).
Acknowledgements
We thank all authors who made data available to us upon request (C. Alaux, K. Antunez, B. Baer, B. Blochtein, C. Botias, B. Dainat, M. Diogon, A. G. Dolezal, V. Doublet, K. Fent, D. C. de Graaf, G. de Grandi-Hoffman, P. Graystock, E. Guzman-Novoa, R. M. Johnson, E. G. Klinger, I. M. de Mattos, N. A. Moran, M. Natsopoulou, F. Nazzi, P. Neumann, R. Odemer, R. Raimets, G. Retschnig, R. M. Roe, E. Ryabov, B. M. Sadd, C. Sandrock, F. Sgolastra, R. Siede, H. V. V. Tome, I. Toplak, S. Tosi, M. Tritschler, V. Zanni and Y. C. Zhu); and J. Bagi and A. J. Folly for helping with the initial screening of titles and abstracts. H.S. was supported by a Royal Holloway University of London Reid PhD Scholarship and by contributions from the High Wycombe Beekeeper’s Association. This project has received funding from the European Horizon 2020 research and innovation programme under grant agreement no. 773921 and ERC Starting Grant BeeDanceGap 638873, and from the Biotechnology and Biological Sciences Research Council, grant/award number BB/N000668/1.
Author information
Authors and Affiliations
Contributions
H.S., E.J.B., C.D.M., T.R.O. and M.J.F.B. conceived the idea for the study in a discussion group. H.S. and E.B. oversaw and managed the data collection. H.S., E.B., C.D.M. and T.R.O. carried out the literature search and collected the data. H.S. and E.L. conducted the statistical analysis and H.S. wrote the first version of the manuscript. H.S., E.J.B., J.K., E.L. and M.J.F.B. contributed to the writing of subsequent drafts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Antica Culina, Adam Vanbergen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Distribution of Hedges’ d values for the individual effect sizes included for the interaction effects of parasites, agrochemicals and nutritional stressors for bee response variables.
a–e, Distributions are shown for mortality (a), behaviour (b), fitness (c), parasite load (d) and immune responses (e). Data are shown as Hedges’ d values ± 95% CI. Effect sizes are sorted for each response variable from most negative to most positive. Each mean ± 95% CI represents a different data point, hence there are more effect sizes than number of studies. Interactions are synergistic when the effect size is positive and the 95% CI does not include zero, antagonistic when the effect size is negative and the 95% CI does not include zero and additive when the 95% CI includes zero. Note that each panel is presented on a different scale.
Extended Data Fig. 2 Hedges’ d values for interactions between specific stressors on bee mortality.
a, Interactions between combinations of parasite stressors. b, Interactions between combinations of parasite and nutritional stressors. Data are shown as Hedges’ d values ± 95% CI. The interactions are synergistic when the effect size is positive and the 95% CI does not include zero, antagonistic when the effect size is negative and the 95% CI does not include zero and additive when the 95% CI includes zero. Numbers next to the 95% CIs indicate the number of effect sizes in each category. Asterisks indicate that the 95% CI does not include zero.
Extended Data Fig. 3 Hedges’ d values for different bee genera.
a–e, Data are shown as Hedges’ d values ± 95% CI for mortality (a), behaviour (b), fitness proxies (c), parasite load (d) and immune responses (e). The genus is indicated by the colour and shape of the symbol. Interactions are synergistic when the effect size is positive and the 95% CI does not include zero, antagonistic when the effect size is negative and the 95% CI does not include zero, and additive when the 95% CI includes zero. Numbers next to the 95% CIs indicate the number of effect sizes in each category. Asterisks indicate that the 95% CI does not include zero. Note that each panel is presented on a different scale.
Extended Data Fig. 4 The interaction effects of different agrochemical classes on bee mortality response measures.
Hedges’ d values ± 95% CI are shown. Asterisks indicate that the 95% CI does not include zero. Numbers next to the 95% CIs indicate the number of effect sizes in each category. Note that effect sizes for azole fungicide × pyrethroid are included in both groups.
Extended Data Fig. 5 Modified PRISMA flowchart.
A flowchart depicting the number of studies included or excluded at each stage of the literature search.
Extended Data Fig. 6 Funnel plots of the full models of the interactions between specific stressors.
a–e, Plots represent the models for mortality (a), behaviour (b), fitness proxies (c), parasite load (d) and immune responses (e).
Supplementary information
Supplementary Information
This file contains Supplementary Text, Supplementary Tables 1-6 and Supplementary References.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siviter, H., Bailes, E.J., Martin, C.D. et al. Agrochemicals interact synergistically to increase bee mortality. Nature 596, 389–392 (2021). https://doi.org/10.1038/s41586-021-03787-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03787-7